


















High efficiency, good sealing, low resistance, long life-span, and light weight, convenient installation and replacement .

Wedge shaped pleat design to prevent the filter media from damage.

Large dust holding capacity and stable performance, cost-effective, affordable.

Low energy consumption, low operating costs. Green environmental protection, no secondary pollution.

Ideal as a pre-filter in air handling units,extends the life of the high efficiency filters.

Used high quality active felt or activated carbon fiber, to meet the special needs of environment
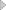
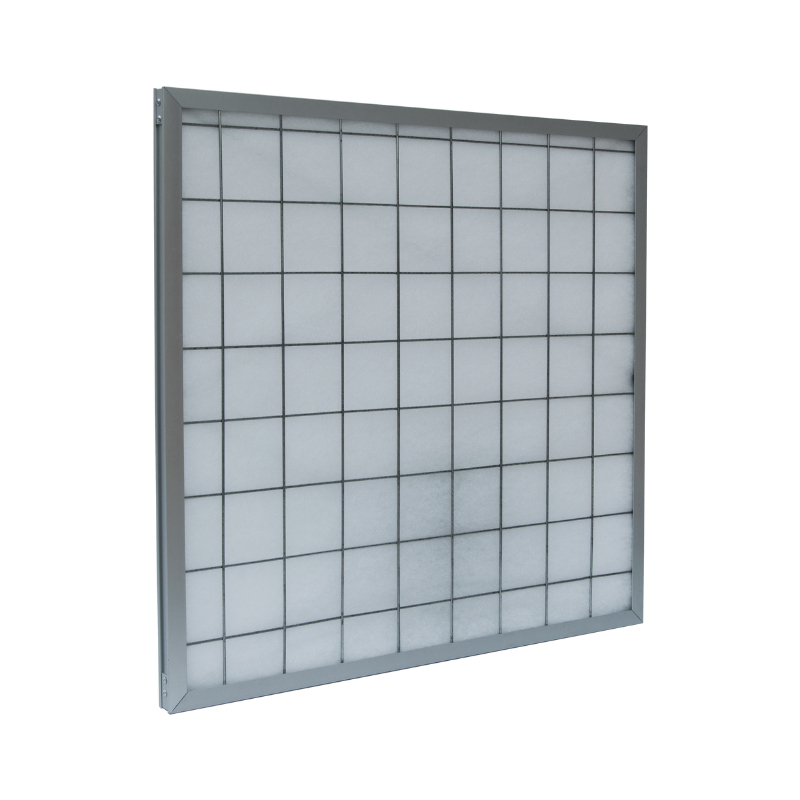
Washable , Replaceable filter media. High strength, large air volume, small resistance, high capacity of dust.

Suitable for acid-based and high humidity-resistance environment.

ISO14644-1 standards, with overheating protection in order to increase users' safety application.
The surface is smooth and bright, lower air resistance and good sound insulation. Special air entrance design and lower the noise effectively.
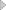

Designers carefully designed the diffuser plate to ensure that the airjet speed to prevent the product from create eddy current.
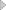

It helps to minimize the risk of cross-contamination from the operator's activities, ensuring the safety of both the operator and the samples or products being handled.
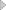

1. Adopts polish stainless steel inside, which is flat, neat and wearable. The surface material of pass box is cold rolled steel or stainless steel.
2. Doors in two sides with mechanical or electronic interlock device enable two doors can't be in the open state at the same time.
3. Pass box with special sealing strips to guarantee the airproof.

♦FREE DESIGN
♦FACTORY PRICE
♦EASY INSTALLATION
♦TIMELY AFTER-SALES SERVICE
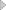

Using leading resistance drain layer technology which allows reasonable airflow distribution inside the clean room , effectively guarantee the indoor cleanliness.
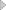

The design is for long distance air supply or discharge, which has advantages of maintenance free, little vibration, low noise and big power air supply. Hanging or floor mounting freely.
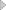

Intelligent control. Adjustable blowing time and interlock system. Strong airproof effect. Stainless steel nozzles at three sides and big air volume. Low noise fan.